electron affinity vs electronegativity|What is the difference between electronegativity and electron : Bacolod Learn the difference between electronegativity and electron affinity, two chemical properties related to electron gain. Electronegativity is the ability to attract electrons, while . Play their legendary endless runner games Temple Run 2: Holi Festival, Temple Run 2: Frozen Shadows, and Temple Run 2: Jungle Fall on Poki! How do you complete Temple Run 2? Collect free coins, jump a second before you near the edges, upgrade your character stats, time your jumps well over double-jump obstacles, and make sure to .
electron affinity vs electronegativity,The increase or decrease of electronegativity depends on whether the sum V fe + (V i + V a)/2 is positive or negative (V fe is the volume of the free electron at given pressure and is always positive); these values are given in SI .Learn the difference between electronegativity and electron affinity, two chemical properties related to atom's ability to attract electrons. Compare their definitio. Learn the difference between electronegativity and electron affinity, two chemical properties related to electron gain. Electronegativity is the ability to attract electrons, while . Learn the definition, trends and examples of electron affinity, the energy change when an atom gains an electron. Compare electron affinity with electronegativity, the ability of .
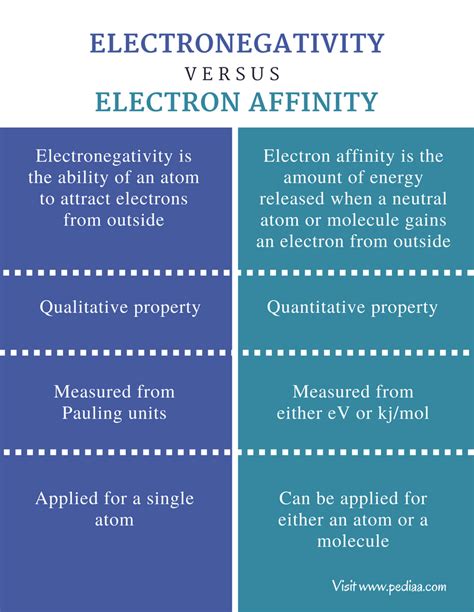
Learn the definitions and differences of electronegativity and electron affinity, two chemical properties related to the attraction of electrons. See examples, formulas, and .Learn how electronegativity affects the polarity of chemical bonds with Khan Academy's video. Explore the trends and patterns of electronegativity in the periodic table. Electron affinity measures the energy change that occurs when an atom gains an electron. 2. Electronegativity is a measure of an atom's ability to pull the shared electrons in . In this blog post, we will explore the concepts of electronegativity and electron affinity, exploring their definitions, differences, and real-life examples. So let’s begin to cover this important topic of the NEET 2024 . The main difference between electronegativity and electron affinity is that electronegativity is the ability of an atom to attract electrons from outside whereas electron affinity is the amount of energy released when an atom .

Learn the difference between electronegativity and electron affinity, two chemical properties related to the movement of electrons. Electronegativity is the attracting ability of an atom, while .
Plotting electron affinity against atomic number displays the trend on the periodic table. (Agung Karjono, CC 3.0) Difference Between Electron Affinity and Electronegativity. Electron affinity and . Well, they are talking about the same thing but here are the definitions. Electronegativity is a chemical property that says how well an atom can attract electrons towards itself. The electronegativity of an atom is influenced by the atom's atomic number and the distance between the atom's valence electrons It was first theorised by Linus Pauling in 1932. .
What is the difference between electronegativity and electron affinity? Answer. Although the trends are both related for similar reasons, electron affinity refers to the energy that is requires to completely gain an electron whereas .

The electron affinity (E ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.. X(g) + e − → X − (g) + energy. This differs by sign from the energy change of electron capture ionization. [1] The electron affinity is positive when energy is released on electron capture.What is the difference between electronegativity and electron The electron affinity (E ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.. X(g) + e − → X − (g) + energy. This differs by sign from the energy change of electron capture ionization. [1] The electron affinity is positive when energy is released on electron capture.The degree to which an atom attracts electrons in a chemical bond is described by electronegativity. If the difference in electronegativity is greater than 1.7, the character of the bond will be ionic. If the difference in electronegativity is between 0.4 and 1.7, the character of the bond is polar covalent.The electron pairs shared between two atoms are not necessarily shared equally. For example, while the bonding electron pair is shared equally in the covalent bond in \(Cl_2\), in \(NaCl\) the 3s electron is stripped from the Na atom and is incorporated into the electronic structure of the Cl atom - and the compound is most accurately described as consisting of individual \(Na^+\) and . Pauling Electronegativity. Exercise \(\PageIndex{1}\) Exercise \(\PageIndex{2}\) References; Linus Pauling described electronegativity as “the power of an atom in a molecule to attract electrons to itself.” 1 Basically, the electronegativity of an atom is a relative value of that atom's ability to attract election density toward itself when it bonds to another atom. As the name suggests, electron affinity is the ability of an atom to accept an electron. Unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gas atom. The more negative the electron affinity value, the higher an atom's affinity for electrons. Figure .
Learn how electronegativity affects the polarity of chemical bonds with Khan Academy's video. Explore the trends and patterns of electronegativity in the periodic table.electron affinity vs electronegativity What is the difference between electronegativity and electron The electron affinity is a measure of the energy change when an electron is added to a neutral atom to form a negative ion. For example, when a neutral chlorine atom in the gaseous form picks up an electron to form a Cl- ion, it releases an energy of 349 kJ/mol or 3.6 eV/atom. . Electronegativity is a measure of the ability of an atom in a .
Electronegativity versus Electron Affinity. We must be careful not to confuse electronegativity and electron affinity. The electron affinity of an element is a measurable physical quantity, namely, the energy released or absorbed when an isolated gas-phase atom acquires an electron, measured in kJ/mol. Electronegativity, on the other hand .The correlation between Mulliken electronegativities (x-axis, in kJ/mol) and Pauling electronegativities (y-axis).Robert S. Mulliken proposed that the arithmetic mean of the first ionization energy (E i) and the electron affinity (E .The Pauling electronegativity scale is based on measurements of the strengths of covalent bonds between different atoms, whereas the Mulliken electronegativity of an element is the average of its first ionization energy and the absolute value of its electron affinity.
electron affinity vs electronegativityThe major difference between electronegativity and electron affinity is that electronegativity is the property associated with the attracting ability of electron towards an atom. As against, electron affinity is associated with the release of energy when an electron is added to an atom.
The electron pairs shared between two atoms are not necessarily shared equally. For example, while the bonding electron pair is shared equally in the covalent bond in \(Cl_2\), in \(NaCl\) the 3s electron is stripped from the Na atom and is incorporated into the electronic structure of the Cl atom - and the compound is most accurately described as consisting of individual \(Na^+\) and .
You should keep in mind that electron affinity is a property of atoms, while electronegativity is a property of atoms when they form bonds with other atoms.. Fluorine is indeed the most electronegative element, which means that if there is a bond between an atom of fluorine and an atom of another element, then the fluorine atom will attract the electrons of the .
The electron pair is screened from both nuclei by the 1s, 2s and 2p electrons, but the chlorine nucleus has 6 more protons in it. It is no wonder the electron pair gets dragged so far towards the chlorine that ions are formed. Electronegativity increases across a period because the number of charges on the nucleus increases. Electronegativity is a measure of how much an atom attracts electrons.For instance, a more electronegative atom will be easily reduced, while a less electronegative atom will be easily oxidized.In covalent bonds, more electronegative atoms "pull harder" on the bonding electrons, so the shared electrons may spend more than half their time with the more .
electron affinity vs electronegativity|What is the difference between electronegativity and electron
PH0 · What is the difference between electronegativity and electron
PH1 · Khan Academy
PH2 · Electronegativity and chemical hardness of elements under pressure
PH3 · Electronegativity and chemical hardness of elements
PH4 · Electron Affinity
PH5 · Difference Between Electronegativity and Electron Affinity
PH6 · Difference Between Electronegativity and Electron Affinity
PH7 · Difference Between Electronegativity and Electron
PH8 · Difference Between Electronegativity And Electron Affinity
PH9 · Difference Between Electronegativity And Electron Affinity
PH10 · Difference Between Electronegativity And Electron